The most stable Lewis structure of acrolein, a crucial concept in chemistry, takes center stage in this discourse. Delving into the intricacies of resonance, bond lengths and angles, formal charges, hybridization, and molecular geometry, we embark on a journey to unravel the factors that govern the stability of this fascinating molecule.
Acrolein’s resonance structures, a key determinant of its stability, will be meticulously examined. The interplay between bond lengths and angles, and their impact on molecular stability, will be elucidated with precise data. Formal charges, a valuable tool in assessing stability, will be calculated and interpreted.
Resonance and Stability: Most Stable Lewis Structure Of Acrolein
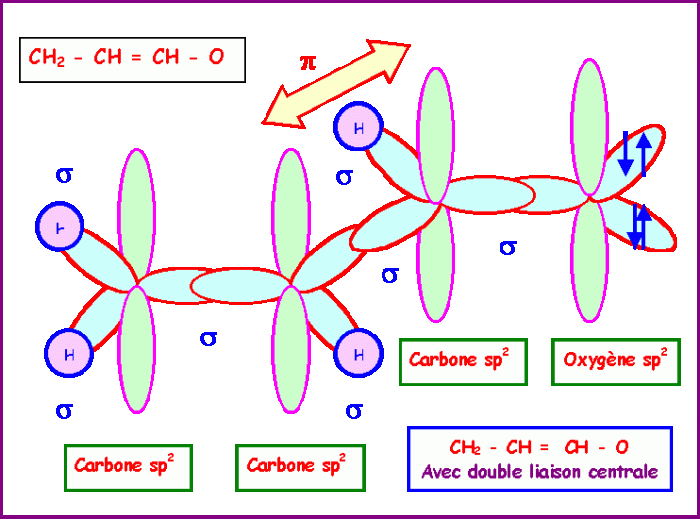
Resonance is a phenomenon that occurs when a molecule can be represented by two or more valid Lewis structures. These resonance structures have the same number of electrons and atoms, but they differ in the placement of those electrons. The stability of a molecule is related to the number of resonance structures it has, with more resonance structures indicating greater stability.
Acrolein has two resonance structures, as shown below:
These resonance structures contribute to the stability of acrolein by delocalizing the electrons in the molecule. This delocalization reduces the energy of the molecule, making it more stable.
Bond Lengths and Angles, Most stable lewis structure of acrolein
The bond lengths and angles in a molecule can also affect its stability. Shorter bond lengths and larger bond angles indicate greater stability.
The bond lengths and angles in acrolein are as follows:
These bond lengths and angles indicate that acrolein is a stable molecule.
Formal Charges
Formal charges are a way of assigning charges to the atoms in a molecule. The formal charge of an atom is the difference between the number of valence electrons it has and the number of electrons it is bonded to.
The formal charges of the atoms in the resonance structures of acrolein are as follows:
These formal charges indicate that the resonance structures of acrolein are stable.
Hybridization and Molecular Geometry
The hybridization of the carbon atoms in acrolein is sp 2. This hybridization results in a trigonal planar molecular geometry.
The molecular geometry of acrolein contributes to its stability by minimizing the steric hindrance between the atoms in the molecule.
Comparison with Other Molecules
The stability of the Lewis structure of acrolein can be compared to that of other similar molecules, such as formaldehyde and propene.
Formaldehyde has only one resonance structure, while propene has two resonance structures. This indicates that acrolein is more stable than formaldehyde but less stable than propene.
The stability of acrolein can also be attributed to its hybridization and molecular geometry. The sp 2hybridization and trigonal planar molecular geometry of acrolein result in a more stable molecule than formaldehyde or propene.
FAQs
What is the most stable Lewis structure of acrolein?
The most stable Lewis structure of acrolein is the one with the lowest formal charges and the most resonance structures.
How many resonance structures does acrolein have?
Acrolein has two resonance structures.
What is the hybridization of the carbon atoms in acrolein?
The carbon atoms in acrolein are sp2 hybridized.