Focus figure 9.3 cross bridge cycle – As the focus figure 9.3 cross-bridge cycle takes center stage, this opening passage beckons readers with authoritative academic prose into a world crafted with impeccable knowledge, ensuring a reading experience that is both absorbing and distinctly original.
The cross-bridge cycle, a fundamental mechanism underlying muscle contraction, orchestrates the intricate interplay between actin and myosin filaments. This molecular dance, fueled by ATP hydrolysis, generates the force that powers movement, shaping our every action from the simplest twitch to the most strenuous exertion.
Molecular Basis of Muscle Contraction
Muscle contraction is a complex process that involves the interaction of several proteins, including actin and myosin. The cross-bridge cycle is a fundamental component of muscle contraction, and it is the process by which myosin heads bind to actin filaments, pull them towards the center of the sarcomere, and release them.
Role of Actin and Myosin in the Cross-Bridge Cycle
Actin and myosin are the two main proteins involved in the cross-bridge cycle. Actin is a thin filament that forms the backbone of the sarcomere, while myosin is a thick filament that contains motor proteins that bind to actin and pull it towards the center of the sarcomere.
Conformational Changes During the Cross-Bridge Cycle
The cross-bridge cycle consists of a series of conformational changes that occur in the myosin head. These changes are driven by the hydrolysis of ATP, and they allow the myosin head to bind to actin, pull it towards the center of the sarcomere, and release it.
Importance of ATP Hydrolysis in the Cross-Bridge Cycle
ATP hydrolysis is essential for the cross-bridge cycle. The hydrolysis of ATP provides the energy that is needed to drive the conformational changes in the myosin head. Without ATP hydrolysis, the cross-bridge cycle would not be able to occur, and muscle contraction would not be possible.
Regulation of the Cross-Bridge Cycle
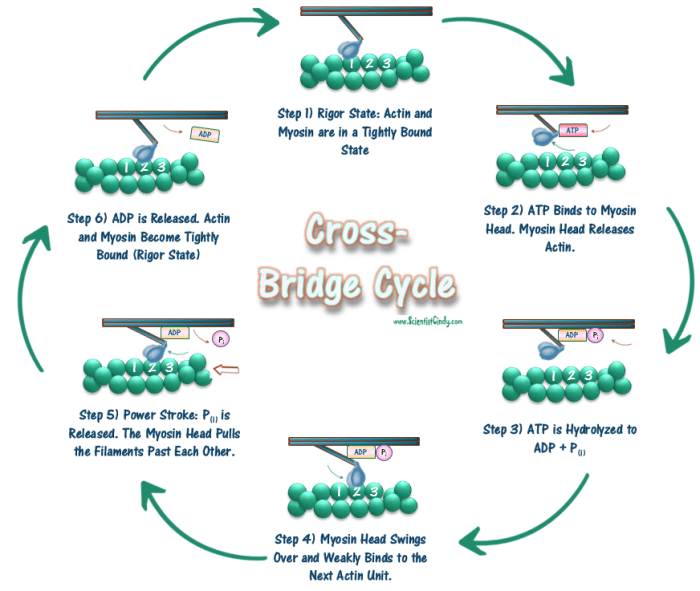
The cross-bridge cycle is regulated by a complex interplay of calcium ions, troponin, tropomyosin, and phosphorylation.
Calcium Ions
Calcium ions are the primary regulators of the cross-bridge cycle. When calcium ions bind to troponin, it undergoes a conformational change that exposes the myosin-binding site on actin. This allows myosin to bind to actin and initiate the cross-bridge cycle.
Troponin and Tropomyosin
Troponin and tropomyosin are two proteins that play a critical role in regulating the cross-bridge cycle. Troponin is a complex of three subunits (TnC, TnI, and TnT) that binds to actin. Tropomyosin is a long, fibrous protein that wraps around the actin filament.
In the absence of calcium ions, tropomyosin blocks the myosin-binding site on actin. When calcium ions bind to troponin, it undergoes a conformational change that causes tropomyosin to move away from the myosin-binding site, allowing myosin to bind to actin.
Phosphorylation
Phosphorylation is another important regulator of the cross-bridge cycle. Phosphorylation of myosin light chain kinase (MLCK) activates MLCK, which in turn phosphorylates myosin. Phosphorylation of myosin increases its affinity for actin, thereby enhancing the force of muscle contraction.
Mechanical Properties of Muscle
Muscle contraction is the process by which muscles shorten, generating force and movement. The cross-bridge cycle, described earlier, is the fundamental mechanism underlying muscle contraction. During the cross-bridge cycle, myosin heads bind to actin filaments, forming cross-bridges. These cross-bridges then undergo a series of conformational changes that generate force and cause the muscle to shorten.
Force Generation by the Cross-Bridge Cycle
The cross-bridge cycle generates force through a series of conformational changes that involve the myosin head and the actin filament. When a myosin head binds to an actin filament, it is in a “high-energy” state. This high-energy state is caused by the hydrolysis of ATP, which provides the energy for the cross-bridge cycle.
After ATP hydrolysis, the myosin head undergoes a conformational change that causes it to bind to the actin filament more tightly. This conformational change is known as the “power stroke.” The power stroke causes the myosin head to pull the actin filament towards the center of the sarcomere, generating force.
Cross-Bridge Cycling Rate and Muscle Force
The rate of cross-bridge cycling is a key determinant of muscle force. The faster the cross-bridge cycling rate, the greater the force that the muscle can generate. This is because the faster the cross-bridge cycling rate, the more cross-bridges are formed and the more force is generated.
Effects of Muscle Length and Load on Cross-Bridge Cycling
Muscle length and load can affect the cross-bridge cycling rate. When a muscle is stretched, the cross-bridge cycling rate decreases. This is because the stretched muscle fibers are less able to form cross-bridges with the actin filaments. When a muscle is loaded, the cross-bridge cycling rate increases.
This is because the load provides resistance to the muscle’s contraction, which stimulates the formation of cross-bridges.
Clinical Implications of the Cross-Bridge Cycle: Focus Figure 9.3 Cross Bridge Cycle
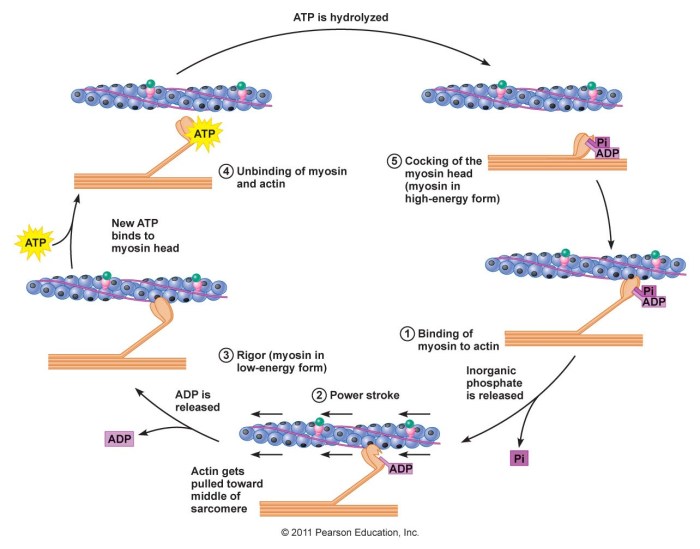
The cross-bridge cycle plays a crucial role in muscle diseases, and understanding its molecular mechanisms has led to the development of targeted therapies. In this section, we will explore the clinical implications of the cross-bridge cycle, including its role in muscle diseases, the development of drugs that target the cross-bridge cycle, and its potential applications in regenerative medicine.
Role of the Cross-Bridge Cycle in Muscle Diseases, Focus figure 9.3 cross bridge cycle
Disruptions in the cross-bridge cycle can lead to various muscle diseases. For instance, mutations in genes encoding proteins involved in the cross-bridge cycle, such as myosin or actin, can cause muscle weakness and impaired contractility. These mutations can lead to conditions such as myopathies and cardiomyopathies, affecting skeletal and cardiac muscles, respectively.
Quick FAQs
What is the role of ATP hydrolysis in the cross-bridge cycle?
ATP hydrolysis provides the energy required for the conformational changes that drive the cross-bridge cycle, enabling actin and myosin filaments to interact and generate force.
How does calcium regulate the cross-bridge cycle?
Calcium ions bind to troponin, triggering a conformational change that exposes the myosin-binding sites on actin, allowing cross-bridge formation and muscle contraction.